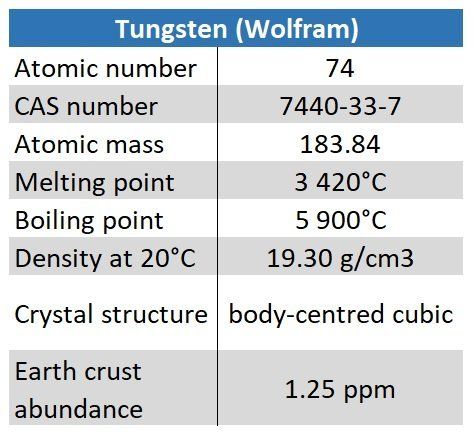
Tungsten (Wolfram)
Tungsten (Wolfram)
Wolframite
History
The name "tungsten" (from the Swedish tung sten, "heavy stone") is used in English, French, and many other languages as the name of the element, but not in the Nordic countries. Tungsten was the old Swedish name for the mineral scheelite. "Wolfram" is used in most European (especially Germanic and Slavic) languages and is derived from the mineral wolframite, which is the origin of the chemical symbol W.[
In 1781, Carl Wilhelm Scheele discovered that a new acid, tungstic acid, could be made from scheelite (at the time named tungsten). Scheele and Torbern Bergman suggested that it might be possible to obtain a new metal by reducing this acid. In 1783 at the Royal Basque Society in the town of Bergara, Spain, the brothers José and Fausto Elhuyar isolated tungsten by reduction of this acid with charcoal, and they are credited with the discovery of the element.
Properties
Tungsten's desirable properties such as resistance to high temperatures, its hardness and density, and its strengthening of alloys made it an important raw material for the arms industry.
Because it retains its strength at high temperatures and has a high melting point, elemental tungsten is used in many high-temperature applications, such as light bulb, cathode-ray tube, and vacuum tube filaments, heating elements, and rocket engine nozzles. Its high melting point also makes tungsten suitable for aerospace and high-temperature uses such as electrical, heating, and welding applications, notably in the gas tungsten arc welding process (also called tungsten inert gas (TIG) welding).
Because of its conductive properties and relative chemical inertness, tungsten is also used in electrodes, and in the emitter tips in electron-beam instruments that use field emission guns, such as electron microscopes. In electronics, tungsten is used as an interconnect material in integrated circuits, between the silicon dioxide dielectric material and the transistors. It is used in metallic films, which replace the wiring used in conventional electronics with a coat of tungsten (or molybdenum) on silicon.
The electronic structure of tungsten makes it one of the main sources for X-ray targets, and also for shielding from high-energy radiations (such as in the radiopharmaceutical industry for shielding radioactive samples of FDG). It is also used in gamma imaging as a material from which coded apertures are made, due to its excellent shielding properties. Tungsten powder is used as a filler material in plastic composites, which are used as a nontoxic substitute for lead in bullets, shot, and radiation shields. Since this element's thermal expansion is similar to borosilicate glass, it is used for making glass-to-metal seals. In addition to its high melting point, when tungsten is doped with potassium, it leads to an increased shape stability (compared to non-doped tungsten). This ensures that the filament does not sag, and no undesired changes occur.
Our products
- Tantalum and Niobium in pure form as well as their alloys
- all common delivery form
China is by far the biggest tungsten producer in the world! (>80% of the global concentrate production)




