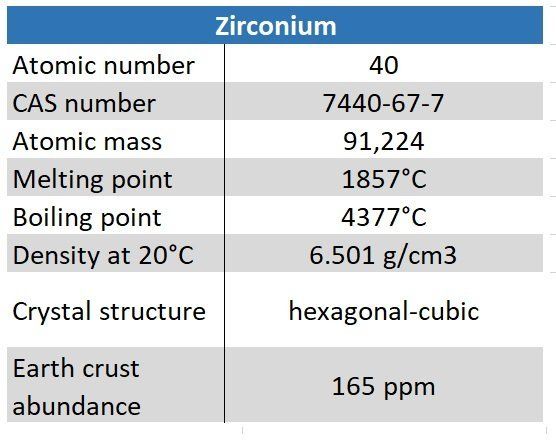
Zirconium
Zirconium
A Zircon Jargoon
History
Zirconium-containing and related minerals were mentioned already in biblical writings. The mineral was not known to contain a new element until 1789 when the German chemist Heinrich Klaproth analyzed a Zircon Mineral stone from the island of Ceylon. He named the new element Zirkonerde (zirconia). Humphry Davy, a cornish chemist, attempted to isolate this new element in 1808 through electrolysis, but failed. Zirconium metal was first obtained in an impure form in 1824 by Jöns-Jacob Berzelius, a Swedish chemist, by heating a mixture of potassium and potassium zirconium fluoride in an iron tube.
The crystal bar process (also known as the Iodide Process), discovered by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925, was the first industrial process for the commercial production of metallic zirconium. It involves the formation and subsequent thermal decomposition of zirconium tetraiodide, and was superseded in 1945 by the much cheaper Kroll process developed by William Justin Kroll, in which zirconium tetrachloride is reduced by magnesium.
The name zirconium is taken from the name of the mineral zircon, the most important source of zirconium. The word zircon comes from the Persian word zargun, that got the meaning "gold-colored.

Properties
Zirconium is a lustrous, greyish-white, soft, ductile, malleable metal that is solid at room temperature, though it is hard and brittle at lesser purities. In powder form, zirconium is highly flammable, but the solid form is much less prone to ignition. Zirconium is highly resistant to corrosion by alkalis, acids, salt water and other agents. However, it will dissolve in hydrochloric and sulfuric acid, especially when fluorine is present. Alloys with zinc are magnetic at less than 35 K.
The melting point of zirconium is 1855 °C (3371 °F), and the boiling point is 4371 °C (7900 °F). Zirconium has an electronegativity of 1.33 on the Pauling scale. Of the elements within the d-block with known electronegativities, zirconium has the fifth lowest electronegativity after hafnium, yttrium, lanthanum, and actinium.
Zirconium is a by-product of the mining and processing of the titanium minerals ilmenite and rutile, as well as tin mining.
For Nuclear usage the Hafnium must be removed from zirconium because hafnium has a high neutron absorption cross-section 600 times greater than zirconium. The separated hafnium can be used for nuclear reactor control rods.
Our products
- Zirconium in pure form and alloys
- all common delivery forms, including composite material (cladded/explosion welded)
- we do not offer Hafnium reduced Zi for nuclear usage!
Special Notes
To export Zirconium from China a export license is needed. Therefore also a end user confirmation has to be provided that confirms a non-nuclear usage of the material. To finalize the paperwork thru all authorities can take 8-12 weeks. Even if we have stock in house this timeframe need to be considered!

